- Product Details
Keywords
- Entecavir High purity
- Hot sale 209216-23-9
- 209216-23-9 suppliers
Quick Details
- ProName: Entecavir High purity Hot sale 209216-...
- CasNo: 209216-23-9
- Molecular Formula: C12H15N5O3
- Appearance: White powder
- Application: API,pharmaceutical raw material
- DeliveryTime: prompt
- PackAge: 10g/bag or as customer's require
- Port: according to details
- ProductionCapacity: Metric Ton/Day
- Purity: 99%
- Storage: sealed and dry preservation
- Transportation: by air/by sea/by courier
- LimitNum: 0 Metric Ton
Superiority
Entecavir High purity Hot sale 209216-23-9 209216-23-9 suppliers
Hunan Warrant Chemical Co., Ltd. is a pharmaceutical research and development and production management. In May 2003, the company was recognized as a high-tech enterprise by the science and technology department of hunan province, and it is also one of the first backbone enterprises in liuyang bio-medicine industrial base of national torch plan.
Since the establishment of the company in 2001, the company has made use of its own capital, talent, information and new drug research and development advantages, on the one hand, focusing on the research and development of new drugs, in a relatively short period of time to complete a series of new drug research and development work. With zhejiang sea is pharmaceutical co., LTD., China medicine group pharmaceutical co., LTD, hainan and brent pharmaceutical co., LTD., keep love some domestic well-known enterprises such as blessed pharmaceutical co., LTD. Signed a number of technology transfer agreement, from 2001 to now realized total technology transfer contract amount is 16.1 million yuan, become a rising star in the field of new drug technology development in hunan province. On the other hand, the company strengthens the construction of production facilities. With the approval of Hunan Provincial Drug Administration, the company has set up a modern production workshop in Liuyang Biological Medicine Park that meets the requirements of GMP. The first-phase project investment is more than 20 million yuan. A solid preparation workshop with an annual production capacity of 200 million tablets, 200 million capsules and 50 million packets of granules and an active drug production workshop with an annual production capacity of 500 tons (mainly producing active drug disodium hydrogen phosphate and sodium dihydrogen phosphate) has been built. In December 2003, all the production workshops of the company have passed the national GMP on-site inspection and obtained the GMP certificate
Our Services
1. Mixed container, we can mix different items in one container.
2. Quality control, with ISO certificate
3. Prompt shipment with professional documents.
4. Packing as your request, with photos before shipment.
5. High quality and competitive price
Details
Product Description
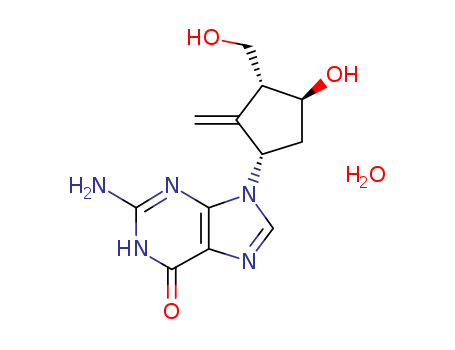
Entecavir is an antiviral nucleoside analog of 2'-deoxyguanosine (Item No. 9002864) and inhibitor of hepatitis B virus (HBV) reverse transcriptase (IC50 = 0.5 nM). It undergoes phosphorylation by cellular kinases to its active form, entecavir triphosphate. Entecavir reduces virion DNA in the culture supernatant of HepG2 2.2.15 cells infected with hepatitis B virus (HBV; EC50 = 3.75 nM). It reduces serum and hepatic levels of viral DNA in a duckling model of HBV infection when administered at a dose of 1 mg/kg. Formulations containing entecavir have been used in the treatment of chronic HBV infection.
Technical Information
| Description |
Entecavir monohydrate (BMS200475 monohydrate; SQ34676 monohydrate) is a potent and selective inhibitor of HBV, with an EC50 of 3.75 nM in HepG2 cell. |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 & Target |
EC50:3.75 nM (anti-HBV, HepG2 cell) |
||||||||||||||||||||
| In Vitro |
Entecavir monohydrate (BMS200475 monohydrate; SQ34676 monohydrate) has a EC50 of 3.75 nM against HBV. It is incorporated into the protein primer of HBV and subsequently inhibits the priming step of the reverse transcriptase. The antiviral activity of BMS-200475 is significantly less against the other RNA and DNA viruses MCE has not independently confirmed the accuracy of these methods. They are for reference only. |
||||||||||||||||||||
| In Vivo |
Daily oral treatment with Entecavir monohydrate at doses ranging from 0.02 to 0.5 mg/kg of body weight for 1 to 3 months effectively reduces the level of woodchuck hepatitis virus (WHV) viremia in chronically infected woodchucks. MCE has not independently confirmed the accuracy of these methods. They are for reference only. |
||||||||||||||||||||
| Clinical Trial |
|
||||||||||||||||||||
| Molecular Weight |
295.29 |
||||||||||||||||||||
| Formula |
C??H??N?O? |
||||||||||||||||||||
| CAS No. |
209216-23-9 |
||||||||||||||||||||
| SMILES |
O=C1NC(N)=NC2=C1N=CN2[C@@H]3C([C@H](CO)[C@@H](O)C3)=C.O |
||||||||||||||||||||
| Shipping |
Room temperature in continental US; may vary elsewhere. |
||||||||||||||||||||
| Storage |
|
||||||||||||||||||||
| Solvent & Solubility |
In Vitro: DMSO : ≥ 50 mg/mL (169.33 mM) H2O : 2.8 mg/mL (9.48 mM; Need ultrasonic and warming) *"≥" means soluble, but saturation unknown. Preparing
*Please refer to the solubility information to select the appropriate solvent. In Vivo:
*All of the co-solvents are provided by MCE. |
Process Capability : The facilities are designed and operated in a manner consistent with GMP,and well maintained at all times in good working order through inspections and the preventive maintenance program.Appropriate cleaning procedures and validation are in place to prevent cross-contamination,especially in facilities where multiple pharmaceutical grade products are processed. Most of our APIs and all formulation production lines are new GMP certified
Entecavir hydrate is an oral antiviral drug used in the treatment of hepatitis B infection. Entecavir hydrate is a nucleoside analog (more specifically, a guanine analogue) that inhibits reverse transcription, DNA replication and transcription in the viral replication process.