- Product Details
Keywords
- Pantoprazole Sodium factory
- Buy 164579-32-2
- 164579-32-2 wholesaler
Quick Details
- ProName: Pantoprazole Sodium factory Buy 164579...
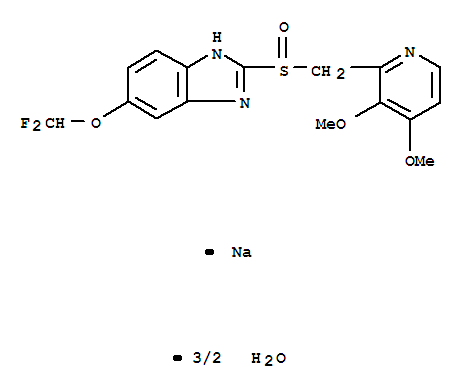
- CasNo: 164579-32-2
- Molecular Formula: C16H15F2N3O4SNa·1.5H2O
- Appearance: Almost white powder
- Application: API, Pharmaceutical raw material
- DeliveryTime: Prompt
- PackAge: 1kg/bag, 25kg/drum
- Port: According to details
- ProductionCapacity: Metric Ton/Day
- Purity: ≥99%
- Storage: Stored in a cool and dry well-closed c...
- Transportation: By air/by sea/by courier
- LimitNum: 0 Metric Ton
- Related Substances: Impurity A NMT 0.20%Impurity B NMT 0.1...
- Heavy Metal: NMT 20ppm
- Valid Period: 2 years
Superiority
Pantoprazole Sodium factory Buy 164579-32-2 wholesaler
Pantoprazole Sodium Hydrate is a metabolite of Pantoprazole Sodium Salt which is an antiulcerative. Gastric pump inhibitor. The free acid, Pantoprazole is a prodrug used in the treatment of acid related disorders and Helicobacter pylori infections.
Our service:
1. High quality with competitive price:
We are manufacturer and we have 2 factories in China,we can provide excellent quality products with factory price.
2. Fast and safe delivery:
1) Parcels can be sent out within 3 days after payment. Tracking number is available.
2) Secure and discreet shipment. You have various choices of transportation methods.
3. We have clients throughout the world:
1) Professional service and rich experience make customers feel at ease, adequate stock and fast delivery meet your desire.
2) Market feedback and goods feedback are appreciated, meeting customers's requirement is our responsibility.
3) High quality, competitive price, fast delivery, first-class service gain the trust and praise from the customers.
4. Exported areas:
Europe , southeast Asia , the Middle East , Africa , South America and some other countries and areas.
5. We manufacture Herbal Extract and Active Pharmaceutical Ingredients products.
Business scope: the pharmaceutical industry with professional import&export services of APIs and Intermediates as well as Herbal Extracts.
Details
Hunan Warrant Chemical Co., Ltd. is a pharmaceutical research and development and production management
In May 2003, the company was recognized as a high-tech enterprise by the science and technology department of hunan province, and it is also one of the first backbone enterprises in liuyang bio-medicine industrial base of national torch plan.
Since the establishment of the company in 2001, the company has made use of its own capital, talent, information and new drug research and development advantages, on the one hand, focusing on the research and development of new drugs, in a relatively short period of time to complete a series of new drug research and development work. With zhejiang sea is pharmaceutical co., LTD., China medicine group pharmaceutical co., LTD, hainan and brent pharmaceutical co., LTD., keep love some domestic well-known enterprises such as blessed pharmaceutical co., LTD. Signed a number of technology transfer agreement, from 2001 to now realized total technology transfer contract amount is 16.1 million yuan, become a rising star in the field of new drug technology development in hunan province. On the other hand, the company strengthens the construction of production facilities. With the approval of Hunan Provincial Drug Administration, the company has set up a modern production workshop in Liuyang Biological Medicine Park that meets the requirements of GMP. The first-phase project investment is more than 20 million yuan. A solid preparation workshop with an annual production capacity of 200 million tablets, 200 million capsules and 50 million packets of granules and an active drug production workshop with an annual production capacity of 500 tons (mainly producing active drug disodium hydrogen phosphate and sodium dihydrogen phosphate) has been built. In December 2003, all the production workshops of the company have passed the national GMP on-site inspection and obtained the GMP certificate
| tem | Specification | Result |
| Identification | I.R similar to standard | Complies |
| The retention time of the major peak corresponds to standard | Complies | |
| Masculine Reaciton for Sodium | Complies | |
| water | Between 5.0% and 8.0% | 5.90% |
| Heavy metals | Not more than 0.002% | Complies |
| Related compounds | Impurity A ≤ 0.20% | 0.08% |
| Impurity B ≤0.15% | Less than detectable limit | |
| Impurity C ≤0.10% | Less than detectable limit | |
| Impurity D & F ≤0.20% | Less than detectable limit | |
| Impurity E ≤0.10% | 0.03% | |
| Any other individual imp ≤0.10% | 0.07% | |
| Total impurities ≤0.50% | 0.18% | |
| Residual solvent | Ethyl acetate ≤0.50% | 0.20% |
| Assay | Between 98.0% and 102.0% | 99.9% |





